Sounds like a very stupid mistake, or? To build a house without access to the roof. Yet this is probably the most common mistake encountered in implant prostheses and the most frequent cause of long-term complications.
by Nikos Mattheos, DDS, MASc, PhD
It’s not the Bacteria, it’s the Biofilm!
A deep sulcus in the interproximal area of anterior implants is the result of the need for natural lookiong aesthetics and paillae.
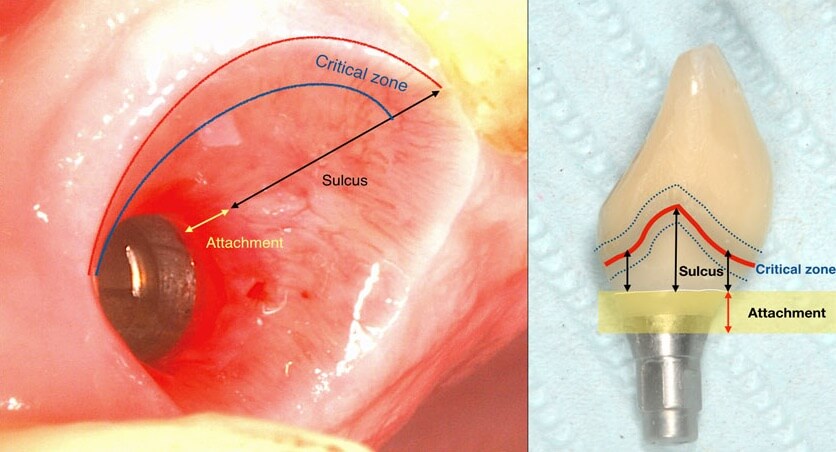
applied at baseline, peri implant tissues with both deep and shallow sulcus remained equally free of inflammation. It is well established since the time of Badersten and Egelberg in the 80’s and 90’s that patient applied oral hygiene will reach no deeper than 0.5-1mm in the sulcus. Yet this was always enough to prevent disease or maintain the outcomes of treatment. So this is where our true battlefield lies: not at the bottom, but at the top of the sulcus, where the prosthesis meets the peri-implant tissues. Disturb the biofilm formation right there, and there will be nothing left to cause inflammation or move apically. Let it accumulate and mature and within a couple of weeks mucositis will be established with the biofilm expanding in all directions, including deeper in the sulcus.
It’s not just the Biofilm, it’s the Emergence Profile!
And this is where we reach the “bottom line” of our investigation: What is the single most likely reason for preventing the biofilm from being removed? You guess right: It’s the prosthesis! And actually, not the entire prosthesis, but this critical circumferential couple of mm that we call “emergence profile”. A study in Sweden by Serino et al, found that the prosthesis was blocking the access to oral hygiene in 48% of the patients diagnosed with peri-implantitis. In my experience with such cases, modification of the prosthesis is an essential step before any attempt to decontaminate the implant. What really strikes me though, is that none of the major published studies I have come across so far in the treatment of peri-implantitis actually mentions anything about prosthesis modification in their protocol. Yet it is essential: Treatment of peri-implantitis begins at the prosthesis.
Treatment of peri-implantitis begins at the prosthesis!
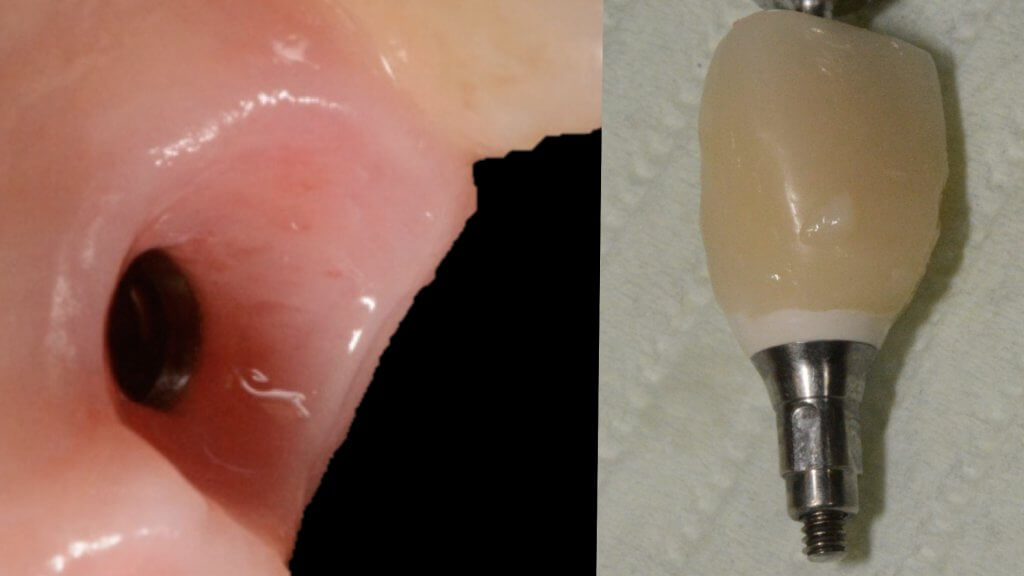
A proper emergence profile is the one that allows direct access to the top of the sulcus and the “danger zone” all around the prosthesis. On the contrary, a typical prosthesis design that blocks access to the sulcus is the “ridge lap”. A ridge lap is a horizontal extension of the prosthesis starting from the top of the sulcus and “riding” the alveolar ridge in mesiodistal or palatolingual direction. We commonly assess the emergence profile in the mesio-distal direction (probably because this is what radiographs show us), but ridge lap is very common (and thus damaging) in the bucco-palatal dimension, too. A recent study by Katafuchi et al, actually showed how the concavity of the emergence profile is important (with concave profile better to convex) and with an emergence angle of more than 30 degrees being significantly correlated with peri-implantitis.
But if nobody likes a ridge lap, why do we keep seeing it so often?
There is only one reason: ridge lap is a compensation for compromised implant selection or position. What are the most common reasons for a ridge lap today:
- a narrow implant platform selected for the replacement of a wide tooth. This is to be perceived both in mesio-distal and palato-lingual dimensions.
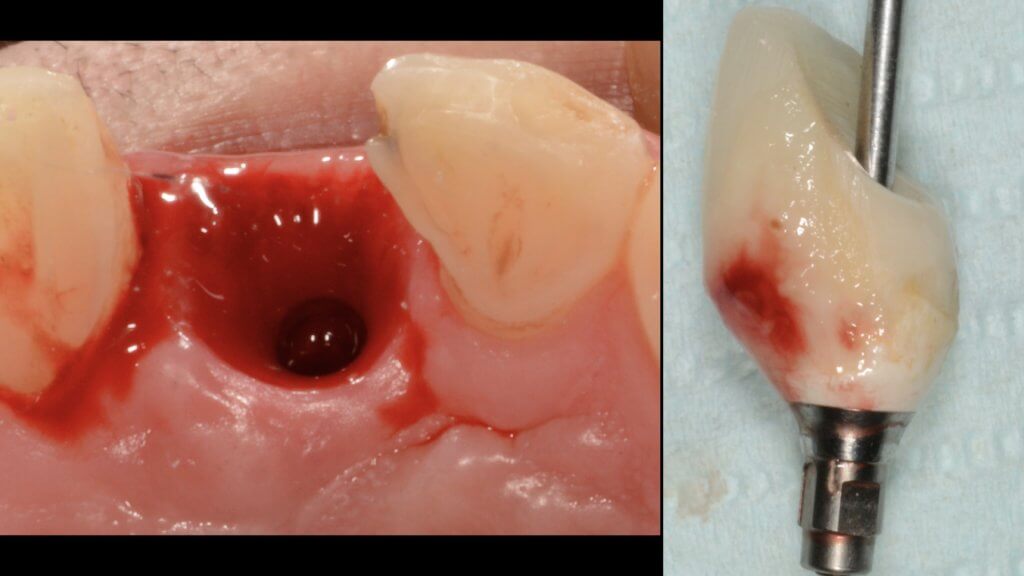
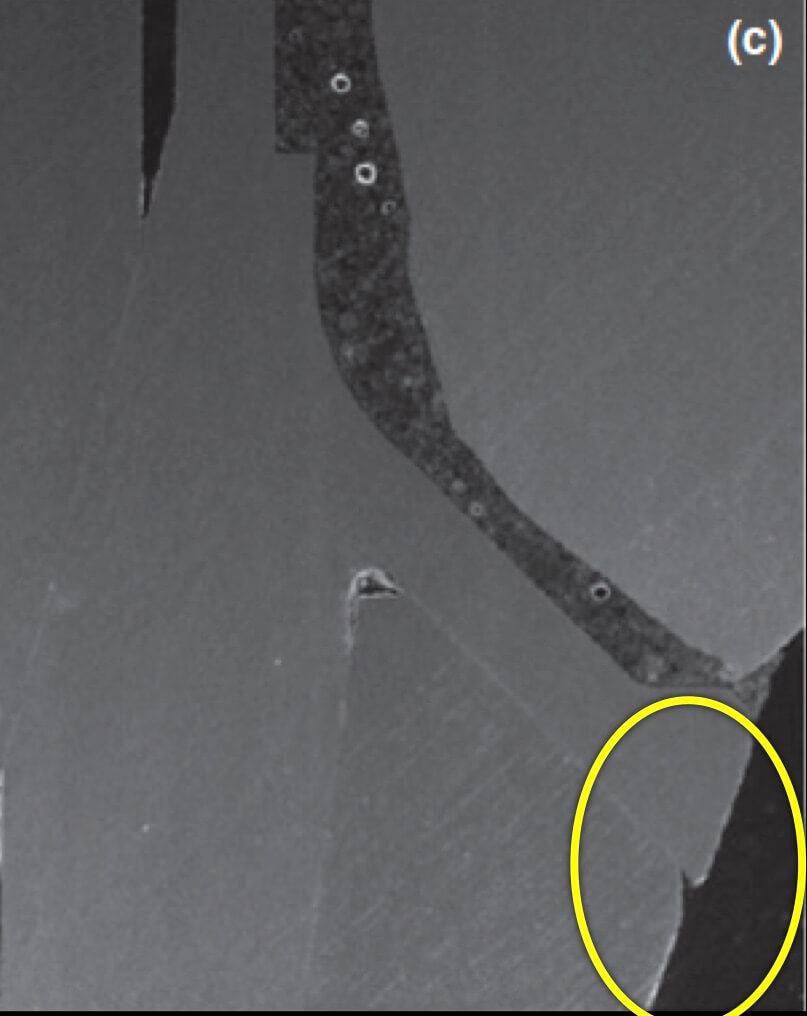
- too shallow implant placement. This is very commonly encountered in the upper anterior sites, where the implant is typically to be placed below the mesial and distal bone margins (a).
- too palatal placement, again very common in the upper anterior sites, where the bone resorption pattern eliminates much of the buccal ridge (b) (Photo courtesy Dr. M Janda).
- too palatal angle of placement (c).
- wrong mesio-distal position (c).
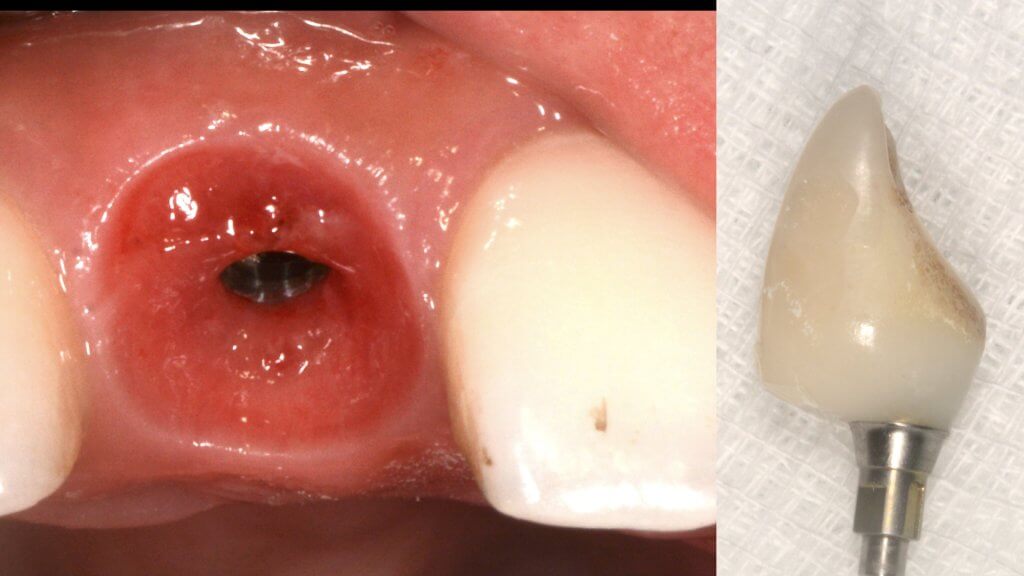
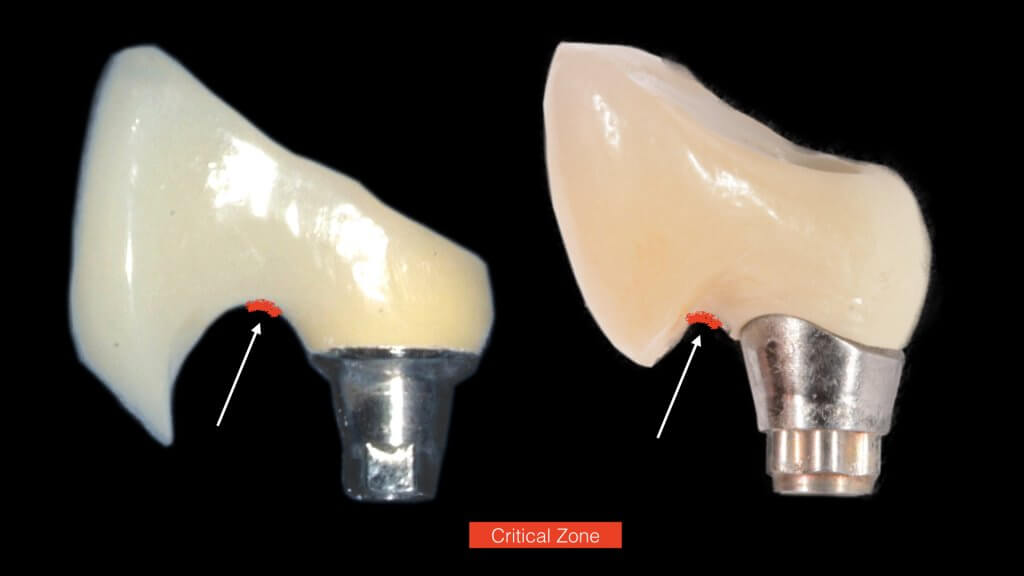
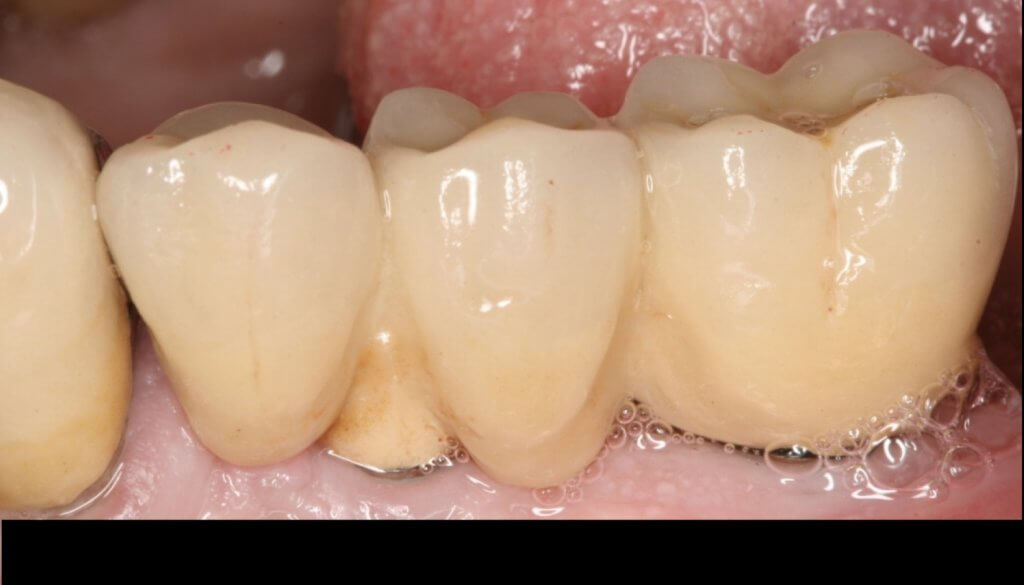
And what if inflammation has already started?
So what if Mucositis kicks in? Well, first thing to do is check the emergence profile: if it blocks access to the sulcus, see if you can modify or change the prosthesis:
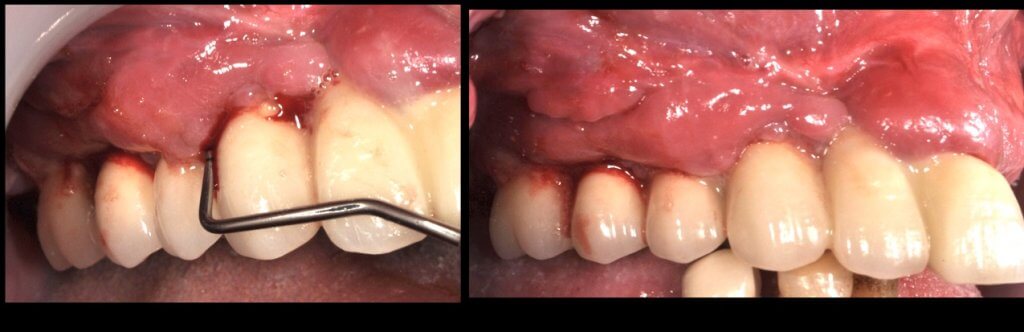
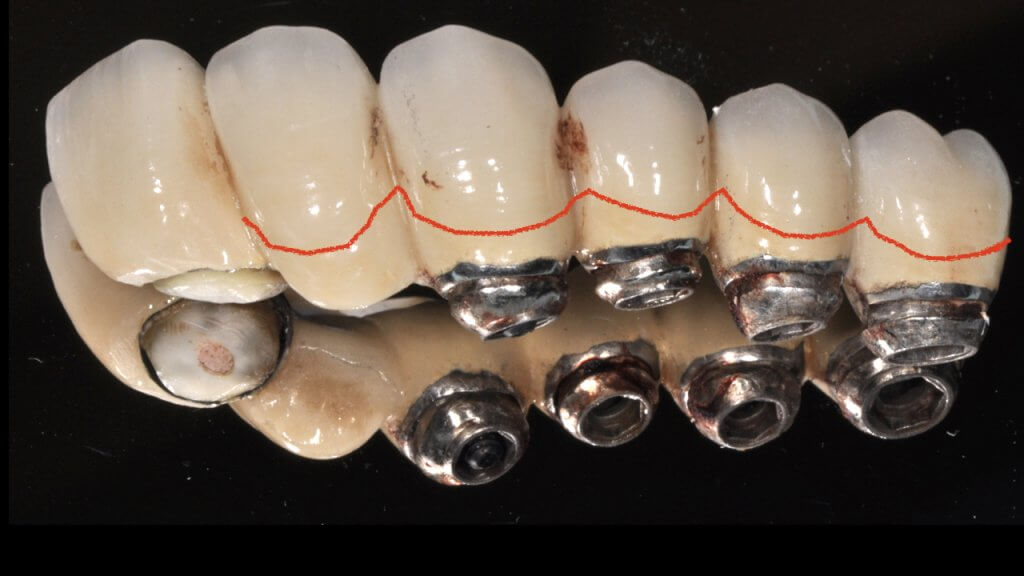
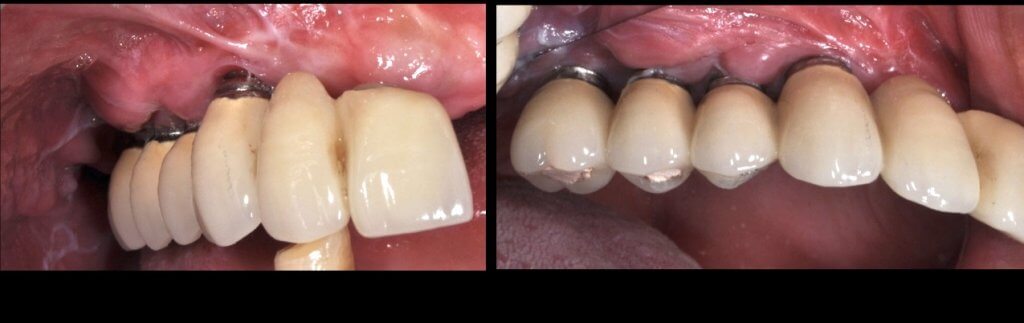
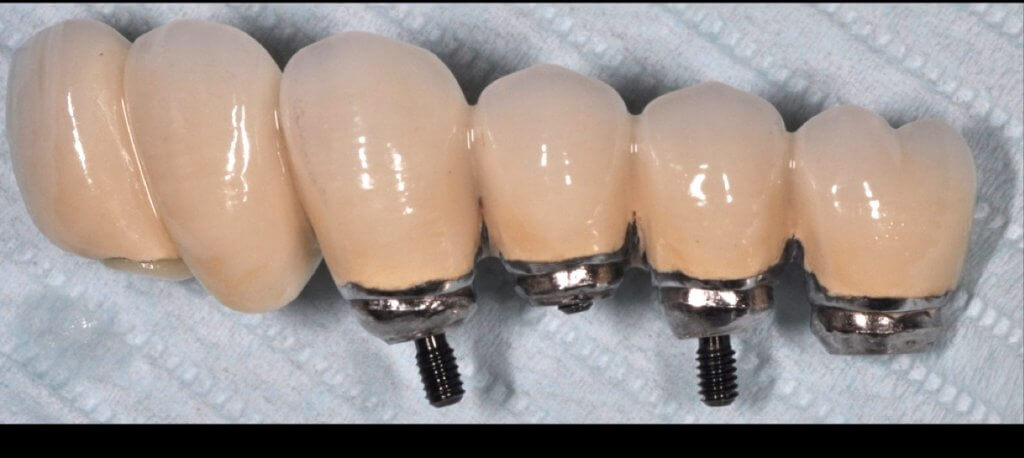
Now what if Mucositis is established at sites with deep sulcus?
The experimental mucositis by Chan et al (4) showed that the inflammation burden is greater in the case of a deep sulcus, which could make pathology progress faster or attempts to treat more difficult. However, there was one more particular parameter in this experiment other than the depth of sulcus: the implants used in that study were all tissue level. As opposed to let’s say UCLA abutments, tissue level implants have a significant “gap” and a “notch” exactly in the position where the implant collar meets the crown, which in the case of deep sulcus will be positioned right at the bottom of the sulcus. Does this matter? Well, let’s talk about this more next time …!
Learn the latest news first, subscribe to my newsletter!
References
- Pontoriero, et al. Experimentally induced peri-implant mucositis. A clinical study in humans. Clin Oral Implants Res. 1994 Dec;5(4): 254-9.
- Zitzmann et al. Experimental peri-implant mucositis in man. J Clin Periodontol. 2001 Jun;28(6):517-23.
- Salvi et al. Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res. 2012 Feb;23(2):182-90.
- Chan D et al. The depth of the implant mucosal tunnel modifies the development and resolution of experimental peri-implant mucositis: A case-control study. J Clin Periodontol. 2019 Feb;46(2):248-255. 7.
7 thoughts on “The roof, the roof, the roof is in fire..! (or shall we say…inflamed!)”
its an amazing article can u email it to me
thank u
Dear Lucy, thank you for your kind comments! This time I was a bit tight with time and did not make a pdf version of the article, but of course please feel free to download it and save it as a pdf yourself if you want! Again, feel free to share and use for any non-commercial purpose!
Nikos
Very nice article and I like your style. I completely agree with you buy but I think a very important aspect of the inflammation of the tissue around implants is the internal micro leakage effect on the increasing the quantity of anaerobic bacteria in the deepest part of the sulcus (specially in bone level implants). In all the implants/prosthesis connections, existed a gap and a pump like effect of bacteria (from the saliva). The anaerobic are selected to survive in the internal cavity of the system, colonize the tunnel and the Teflon or the cotton or any material the prostodontist use to seal the tunnel.
That’s why I invented the SilverPlug (as you know). The silver reduce the amount of bacteria inside the system and helps the control of the quality of the biofilm. http://Www.silverplug.ch
Thank you for your insight! I do understand the problem, which can be a significant threat at times, depending on the implant system and the reconstruction type. In the past for example with older type external connections, this leakage might have been one of the main reasons why bone was always “running away” from the implant shoulder. Having sliced some of the modern internal connections under the SEM, the gap of a bone level with the abutment is less than 2-3 μm which would minimise the possibility of leakage. Then again recently the trend is to use ceramic restorations cemented on titanium “base” abutments, which introduced the risk of a new “gap” in the sulcus, this time between the ceramic crown and the Titanium base… So I think you are right, we need to seriously evaluate the risks of micro-leakage and act accordingly! thank you for your comments!
I love your article Nikos. It paints a very clear picture.
Many thanks Charles!
Hi! This post couldn’t be written any better! Reading this post reminds me of my old room
mate! He always kept talking about this. I will forward this write-up to him.
Pretty sure he will have a good read. Thank you for sharing!